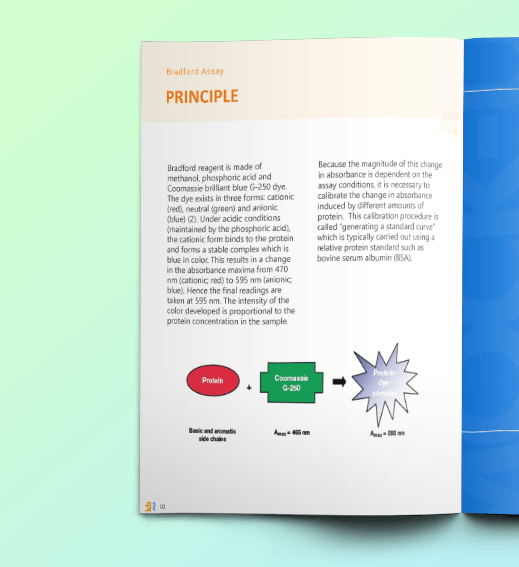
Protein Concentration SOP: Bradford Assay

SOP: Protein Reagent Concentration
Determining Sample Consistency
Whether your lab already has an SOP in place for new protein analysis or would benefit from a revised procedure, we provide a simplified version of kbDNAs in-house Bradford Assay protocol for concentration testing commercial protein reagents.
Why concentration and why now?
> LAB REPLENISHING
Many researchers have begun ordering recombinant reagents as part of their material replenishing & it's important to revisit the role of concentration
> COVID IMPACT
Due to the unique disruption in the manufacturing sector, the likelihood of receiving proteins from the same batch (or lot) prior to COVID is low
> CONCENTRATION FACTOR
Measuring sample concentration and comparing it to the data on the certificate of analysis is the most direct way to determine whether any quality was impacted
> VERIFYING QUALITY
We suggest running Bradford assay tests on both same and different lot samples since the disruption could have occurred during the production or storage period
















