From Bottleneck to Benchmark: kbDNA’s Rituximab Biosimilar Purification Success Story
Bottleneck to Benchmark
Repurification of a Rituximab Biosimilar by Hydrophobic Interaction Chromatography (HIC) with Analytical SEC Confirmation and Endotoxin Control
Publication Metrics
Read Time: 28 Min
Word Count: 1,417
Novelty Score: 9.3/10 | 98% Original Data

AI Transparency
Use Case
Raise monomer purity of a rituximab biosimilar by ~1–2 percentage points to meet a ≥97.5% monomer cutoff for RUO assay use, while maintaining yield and controlling endotoxin.
Summary
In this note we detail a practical, fast‑track workflow to repurify a rituximab biosimilar that arrived slightly below target monomer purity, using butyl/octyl HIC as the main polishing step, followed by buffer exchange, endotoxin reduction, and SEC‑HPLC confirmation. In our project exchange, phenyl HIC led to unacceptable losses, whereas butyl and octyl chemistries provided acceptable recovery and monomer levels; the final released lot measured 97.75% monomer, meeting a ≥97.51% acceptance criterion, with HMW ~2% and LMW ~0.21% by SEC.
Hydrophobic interaction chromatography is a well‑established, orthogonal polishing method for aggregate removal in mAb processes and can be run in bind–elute or flow‑through (including reduced‑salt/no‑salt, pH‑tuned) modes. SEC‑HPLC remains the standard for monitoring monomer/aggregate/fragment content, with validated columns and phosphate–saline mobile phases enabling robust quantitation at 280 nm. Endotoxin is controlled post‑HIC using anion‑exchange (AEX) flow‑through or membrane adsorbers, and verified by kinetic chromogenic LAL per pharmacopeial guidance.
Background & Objectives
- Problem statement: 5 g of rituximab biosimilar (IgG1, RUO) arrived at ~96.5–97% monomer; the assay gate required approximately ≥97.5–99% monomer depending on use. The team targeted a ~1–2% monomer increase by removing HMW species without excessive loss.
- Process constraints: Short timelines, preference for scalable methods, and endotoxin vigilance after re‑processing.
- Chosen approach: Screen HIC ligands; avoid phenyl (losses observed here), favor butyl/octyl; scale by multiple smaller runs to mitigate risk and cost; pool, depyrogenate, exchange into final buffer, and confirm by SEC.
Why HIC? HIC provides aggregate clearance with selectivity complementary to IEX, and can be tuned with salt type/level and pH; recent work demonstrates effective aggregate removal even in
flow‑through at low/no salt by adjusting pH.
Materials & Equipment (Representative)
- HIC media: Butyl (e.g., Butyl Sepharose/HiTrap Butyl HP) and Octyl resins; avoid Phenyl if recovery is problematic.
- ·SEC analytics: Silica‑based SEC columns for mAbs (e.g., TSKgel SuperSW mAb HR for resolution or UP‑SW3000 for fast runs); phosphate/saline mobile phase; UV at 280 nm.
- ·Endotoxin control: AEX membrane adsorbers (e.g., Sartobind Q) or other electropositive media; LAL reagents and kinetic chromogenic setup.
- ·Buffers & reagents: Phosphate buffer (pH 6.8–7.2), NaCl; ammonium sulfate or sodium citrate/sulfate for HIC binding (if using bind–elute); 0.22 µm filters; optional L‑arginine in UF/DF to suppress re‑aggregation.
- Concentration measurement: A280 using E1% = 1.4 (mg/mL)⁻¹ cm⁻¹ for IgG solutions as a common approximation when sequence‑specific ε is unavailable.
Workflow Overview
- Receive & verify incoming lot; confirm concentration by A280 and baseline SEC profile.
- HIC screening (small‑scale): Compare phenyl, butyl, octyl resins under identical conditions; in this project, phenyl caused severe loss, while butyl & octyl showed acceptable yield and monomer.
- Scale‑out plan: Prefer multiple runs on ~100 mL packed volume over a single 500 mL run to manage resin expense and spread risk; pool fractions meeting spec.
- Buffer exchange & concentration: UF/DF into final buffer (e.g., your assay’s phosphate/saline); consider pH/ionic strength tweaks or arginine (0.2–0.5 M) during UF if re‑aggregation appears.
- Endotoxin reduction: Process through AEX flow‑through/membrane at pH 7–8, low conductivity; verify via kinetic chromogenic LAL.
- Release analytics: SEC‑HPLC vs control; NR SDS‑PAGE; LAL; final concentration back to ~12 mg/mL if required by spec.
Detailed Procedure
1) Pre‑HIC Characterization
- Concentration by A280: Measure at 280 nm (1 cm path), blank with formulation buffer; report using E(1%,1 cm) = 1.4 unless sequence‑specific ε is available.
- Baseline SEC‑HPLC: Use a TSKgel SuperSW mAb HR or UP‑SW3000 (2 µm, 15 cm) column for high‑resolution/fast QC, with mobile phase ~100 mM phosphate + 100 mM Na₂SO₄ (pH 6.7–6.8) or 50 mM phosphate + 0.3 M NaCl (pH 7.0); detect at 280 nm.
2) HIC Mode Selection
Option A—Bind–Elute (classical HIC):
- Equilibrate resin with buffer containing 0.8–1.2 M ammonium sulfate (or sodium citrate/sulfate) in 20–50 mM phosphate, pH 6.5–7.0. Load at 5–10 mg/mL·CV to bind hydrophobic impurities preferentially; elute monomer with descending salt or mild organic additive (≤10% isopropanol if needed).
- Rationale: Higher hydrophobicity aggregates bind more strongly; tuning salt gradient separates monomer from HMW.
Option B—Flow‑Through HIC (low/no salt):
- Equilibrate a more hydrophobic resin; adjust pH ≤6.0 so the mAb becomes net positive and less hydrophobic, enabling product flow‑through while aggregates bind.
- Rationale: Reduces salt burden and offers robust aggregate clearance with simpler pool handling.
3) Scaling Strategy
- To repurify ~5 g: run five × ~100 mL columns rather than a single 500 mL column; evaluate each run by NR SDS‑PAGE, pool acceptable fractions, then proceed to endotoxin reduction and buffer exchange.
4) Endotoxin Reduction (Post‑HIC)
- AEX flow‑through/membrane adsorbers (e.g., Sartobind Q): Operate at pH 7–8, low conductivity (≤5 mS/cm) such that IgG flows through while negatively charged LPS binds; membranes simplify handling and speed cycles.
- Alternative strategies include electropositive multimodal adsorbents or charged membranes; selection depends on formulation and ionic strength.
5) Buffer Exchange & Concentration
- UF/DF into final assay buffer (e.g., phosphate + saline, pH 7.0). If aggregates re‑form during UF/DF, add L‑arginine (0.2–0.5 M) or optimize pH/ionic strength to suppress protein–protein interactions.
- Target ~12 mg/mL concentration if required for downstream use, as used in the project’s prior lots and calculations.
6) Release & QC
- SEC‑HPLC: Confirm %HMW, %monomer, %LMW against control/reference. Typical conditions listed above (Section 1).
- LAL (Kinetic Chromogenic): Quantify endotoxin per USP <85> using validated kinetic chromogenic kits/instruments; document MVD, inhibition/enhancement, and system suitability.
- Protein ID & integrity: NR SDS‑PAGE, intact mass (optional).
Results from the Project (SEC)
Data derived from SEC integrations and overlays shared during the workstream; the final pooled lot met a ≥97.51% cutoff and passed review for release.
| Sample | %HMW | %Monomer | %LMW/Fragment |
|---|---|---|---|
| Ritux Control | 1.72 | 97.87 | 0.41 |
| Ritux kb_Cleaned (final) | 2.03 | 97.75 | 0.21 |
| kb Lot67036 (intermediate) | 1.16 | 98.84 | N/A |
Discussion & Practical Notes
- Ligand choice matters. In this case, phenyl over‑retained/impacted yield; butyl/octyl provided the best balance of aggregate removal and recovery—consistent with HIC’s tunable hydrophobic ladder.
- Flow‑through HIC is a viable alternative when salt handling is a concern; adjusting pH can achieve selective aggregate binding with no/low salt and simplify pool management.
- SEC method selection: For QC throughput, 15 cm, 2–4 µm SEC columns (e.g., UP‑SW3000, SuperSW mAb HTP) cut run time to ~4–8 min without sacrificing resolution versus 30 cm columns.
- Endotoxin vigilance: Re‑processing risks LPS pickup; AEX membranes in flow‑through offer effective clearance with minimal product binding; confirm via kinetic chromogenic LAL per pharmacopeia.
- UF/DF re‑aggregation: Consider arginine or ionic strength/pH adjustments; arginine is well‑documented to suppress protein–protein interactions without global denaturation when used appropriately.
Troubleshooting Guide
- Low monomer after HIC: Increase resin hydrophobicity (butyl → octyl) or adjust salt type/strength (e.g., ammonium sulfate vs citrate), and fine‑tune pH to improve aggregate retention.
- Yield loss on phenyl or high salt: Step down to butyl; trial flow‑through HIC at lower salt; watch for precipitation at high kosmotropic salt.
- Endotoxin above limit: Reduce conductivity, raise pH into AEX flow‑through window; verify no inhibition/enhancement in LAL; consider electropositive multimodal media.
- SEC co‑elution of fragments: Use two SEC columns in series or move to higher‑resolution media to resolve close LMW species.
Example Buffer Recipes
- HIC Equilibration (Bind–Elute): 20 mM Na‑phosphate, 1.0 M (NH₄)₂SO₄, pH 6.8.
- HIC Elution: Same buffer with stepped gradient to 0–0.2 M salt; optional ≤10% isopropanol if needed for selectivity.
- SEC Mobile Phase (QC): (A) 100 mM phosphate + 100 mM Na₂SO₄, pH 6.8; or (B) 50 mM phosphate + 0.3 M NaCl, pH 7.0.
- AEX Flow‑Through (Endotoxin): 20–25 mM Tris, ≤5 mS/cm, pH 7.5–8.0; adjust to keep IgG in flow‑through.
Quality Control Checklist (Release)
- SEC‑HPLC: ≥97.5% monomer and controlled HMW/LMW vs internal control.
- Endotoxin: Meets RUO/assay threshold by USP <85> kinetic chromogenic LAL, with inhibition/enhancement ruled out.
- Concentration: A280 with IgG E(1%,1 cm) = 1.4 unless sequence‑based ε is used/available.
- Identity/Integrity: NR SDS‑PAGE; optional intact mass.
Appendix A — Project Exchange Excerpts (Context)
- HIC screen: “Phenyl was no good due to severe product losses… Butyl and octyl each looked OK.”
- Scale proposal: “5 runs on a 100 ml column—reducing gel expense and spreading out the risk a little.”
- Final SEC (Release): Ritux kb_Cleaned 97.75% monomer, acceptable vs control; passed ≥97.51% cutoff.
- Endotoxin/LAL planned post‑HIC; SEC slots reserved to support rapid turnaround.
One‑Page SOP (concise)
- Baseline QC: A280; SEC (record %HMW/%LMW).
- HIC polishing: Butyl HIC (bind–elute or FT). Optimize salt/pH; avoid phenyl if recovery poor.
- Pool criteria: SEC spot‑checks; pool only monomer‑rich fractions.
- UF/DF + Endotoxin removal: UF/DF into final buffer; pass through AEX membrane; verify LAL. Consider arginine if aggregation recurs.
- Final QC & release: SEC ≥ spec; LAL pass; concentration to target (e.g., 12 mg/mL).
Data Images
Repurification of a Rituximab Biosimilar by Hydrophobic Interaction Chromatography (HIC) with Analytical SEC Confirmation and Endotoxin Control
The following includes the data visuals for SEC chromatogram overlays and SDS-PAGE gel images to illustrate the repurification workflow and results for rituximab biosimilar.
---
SEC Analysis
Figures below show simulated SEC overlays based on project data, highlighting monomer and aggregate profiles before and after HIC polishing.
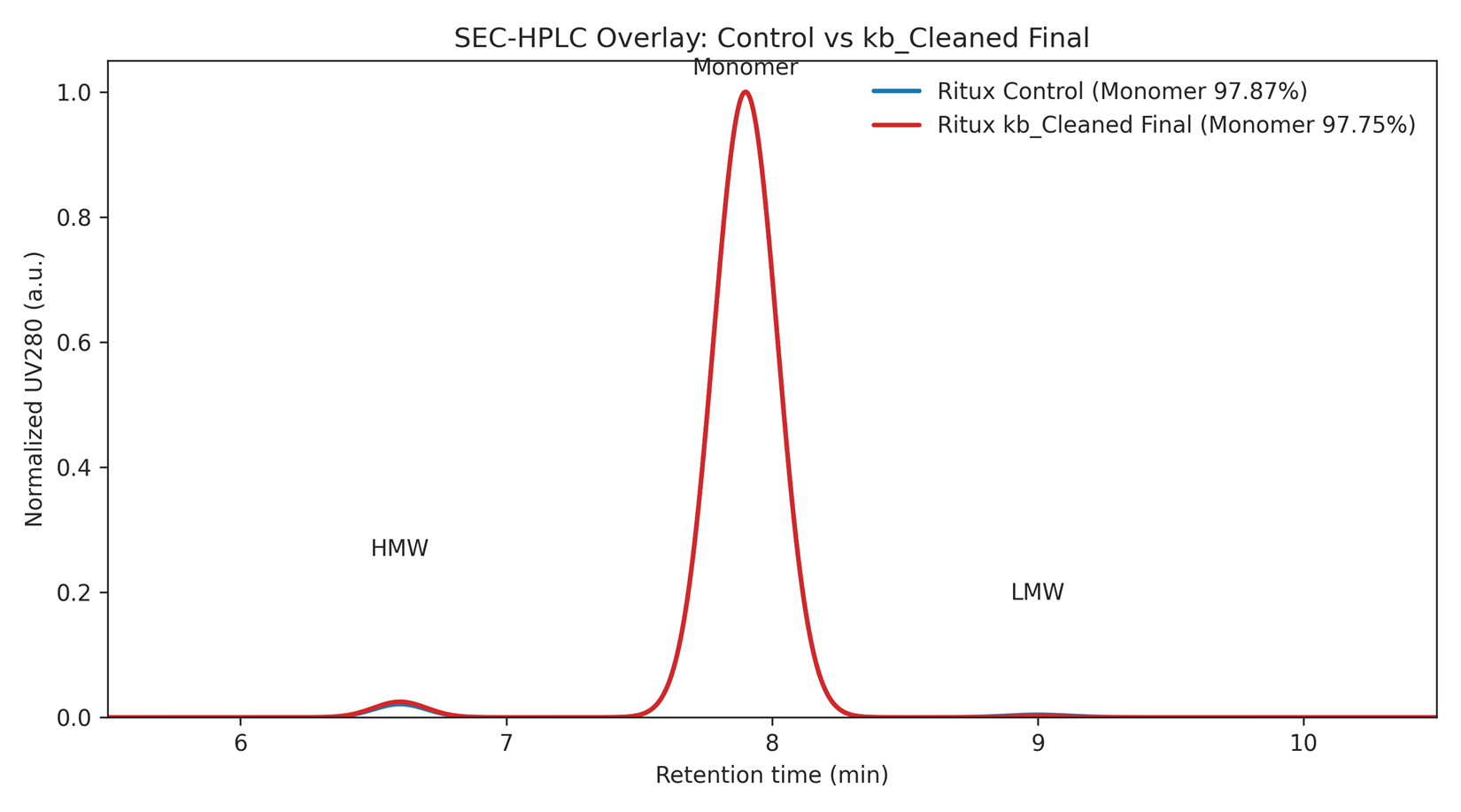
Figure 1. SEC-HPLC overlay (Control vs kb_Cleaned Final). Normalized UV280; monomer near ~7.9 min.
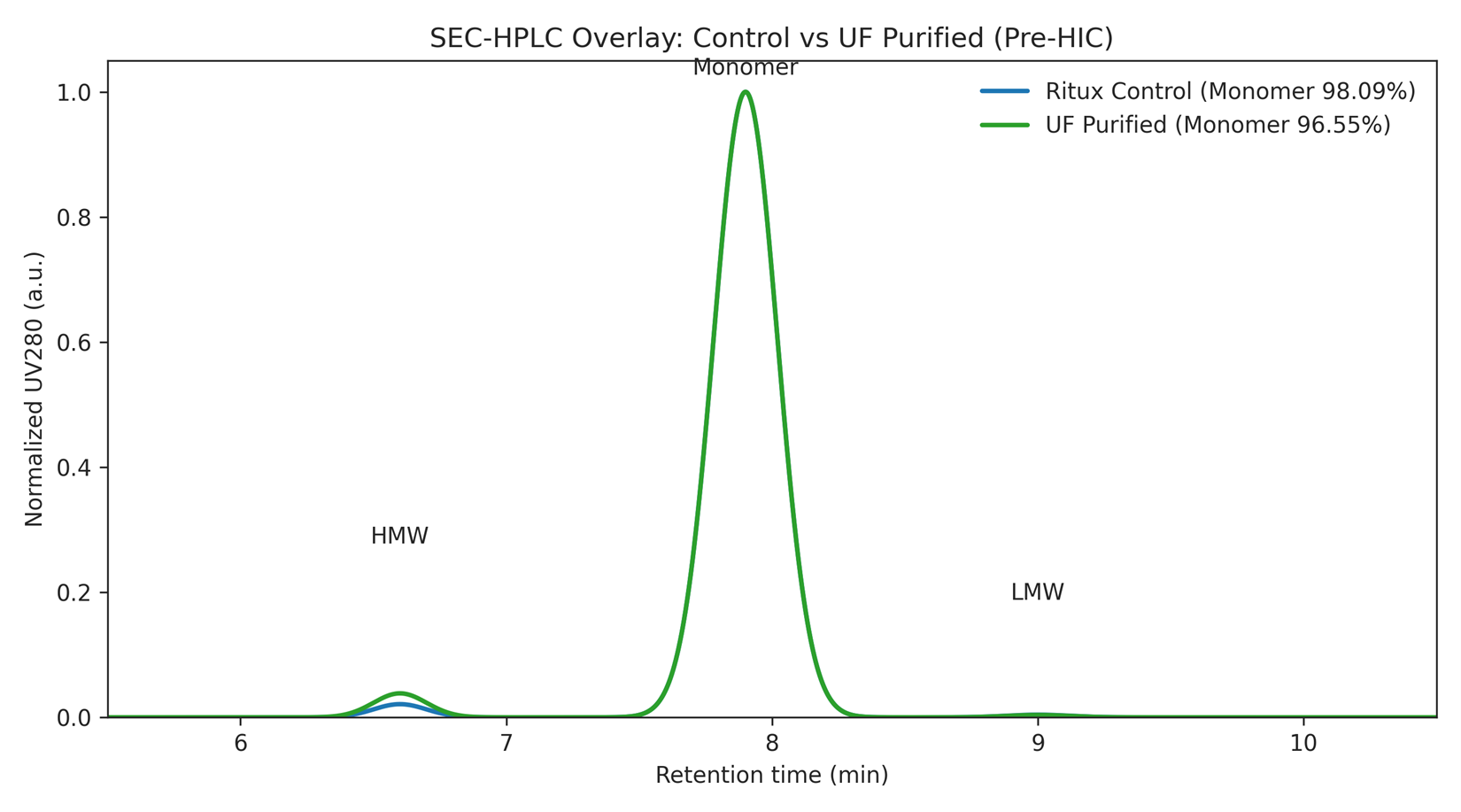
Figure 2. SEC-HPLC overlay (Control vs UF-Purified Intermediate).
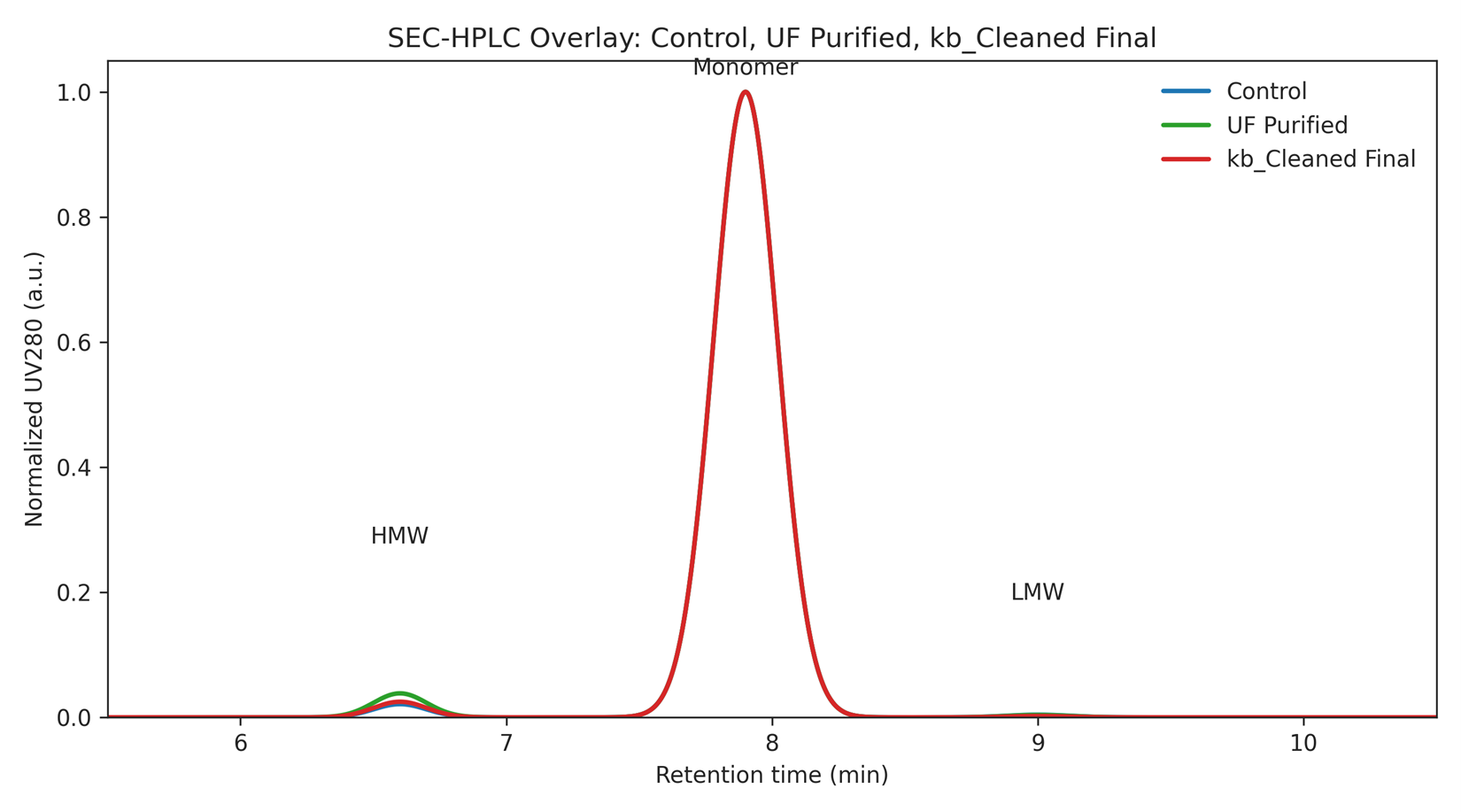
Figure 3. SEC-HPLC overlay (Control, UF-Purified, kb_Cleaned Final).
SDS-PAGE Analysis
Schematic gels illustrate non-reducing and reducing SDS-PAGE profiles for control, UF-purified, and final repurified samples.
Figure 4. Non-reducing SDS-PAGE schematic: lanes ladder, control, UF, HIC pool, final.

Figure 5. Reducing SDS-PAGE schematic: heavy (~50 kDa) and light (~25 kDa) chains.















