Ensuring Safe and Secure Nucleic Acid Procurement: A New Framework
kbDNA Leads the Charge
In Compliance and Security
The scientific community is on the brink of a transformative era with the introduction of the new Framework for Nucleic Acid Synthesis Screening. Published by the Fast Track Action Committee on Synthetic Nucleic Acid Procurement Screening under the National Science and Technology Council (NSTC), this comprehensive framework aims to mitigate risks associated with the misuse of synthetic nucleic acids, bolstering biosecurity and ensuring the beneficial use of these critical building blocks of life.
Understanding the New Framework
The framework, mandated by the Executive Order issued in October 2023, focuses on reducing the risks of synthetic nucleic acid misuse and enhancing biosecurity measures. It outlines a unified process for screening purchases of synthetic nucleic acids and bench-top synthesis equipment. The guidance targets providers of synthetic nucleic acids and manufacturers of bench-top nucleic acid synthesis equipment, encouraging them to implement thorough, scalable, and verifiable procurement screening mechanisms. The framework is based on the following principles:
- Risk-based: The screening process is tailored to the level of risk posed by the synthetic nucleic acid or the bench-top synthesis equipment, taking into account factors such as the sequence, the quantity, the customer, and the destination.
- Transparent: The screening process is clear and consistent, allowing providers and manufacturers to understand their obligations and customers to know what to expect.
- Collaborative: The screening process involves coordination and communication among stakeholders, including federal agencies, industry associations, academic institutions, and international partners.
- Adaptive: The screening process is flexible and responsive to emerging threats and technological developments, allowing for continuous improvement and innovation.
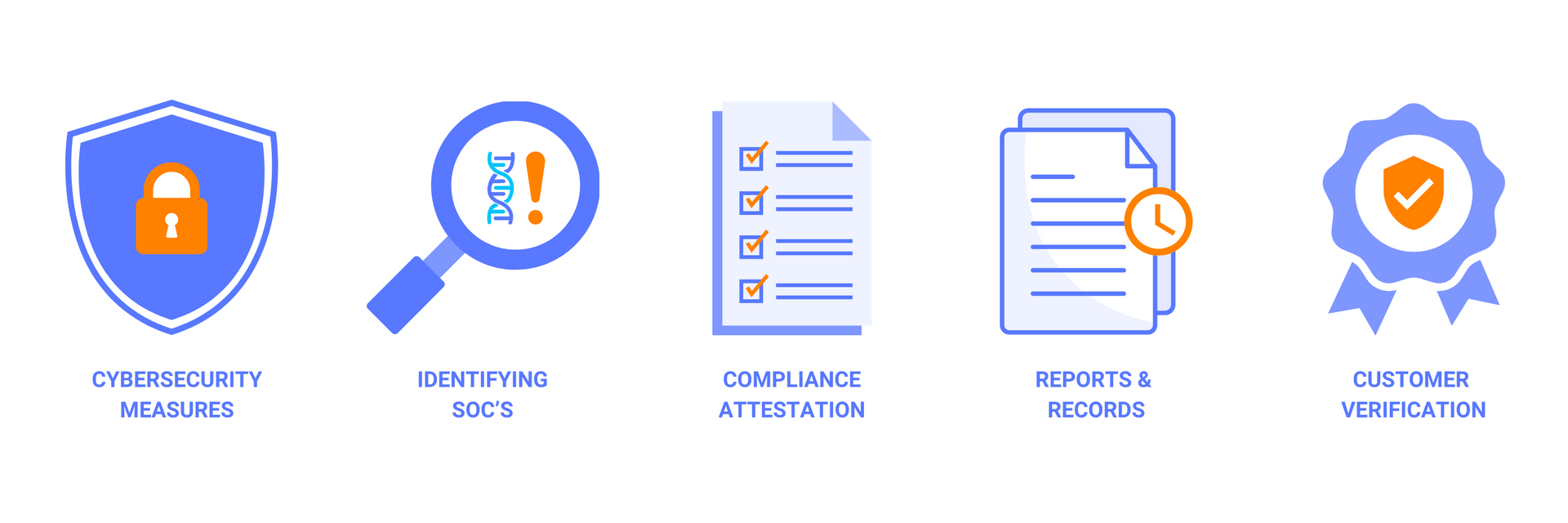
Key Aspects of the Framework
- Attestation of Compliance: Providers and manufacturers must attest to implementing the screening framework, either publicly or directly to customers and federal funding agencies.
- Identification of Sequences of Concern (SOCs): Purchase orders for synthetic nucleic acids must be screened to identify SOCs, ensuring that potentially harmful sequences are flagged and managed appropriately. SOCs are defined as sequences that are known or reasonably suspected to pose a biosecurity risk, such as those that encode for toxins, pathogens, or bioweapons.
- Verification of Customer Legitimacy: Customers placing orders for synthetic nucleic acids or benchtop synthesis equipment must undergo legitimacy checks, verifying their institutional affiliations and intended use. Legitimacy checks may include requesting documentation, contacting references, or conducting site visits.
- Reporting and Record Retention: Providers and manufacturers are required to report flagged orders and retain records related to the purchase orders for at least three years. Reporting may involve notifying relevant federal agencies, law enforcement authorities, or international organizations. Record retention may include storing information on the sequence, the customer, the screening outcome, and the actions taken.
- Cybersecurity Measures: Information security and cybersecurity practices are paramount, especially for SOC databases that hold sensitive biosecurity data. Providers and manufacturers must ensure that their data is protected from unauthorized access, modification, or disclosure, using encryption, authentication, and backup systems.
kbDNA's Commitment to Excellence
At kbDNA (kbdna.com), we are proud to be at the forefront of compliance and security in the procurement of synthetic nucleic acids. Our company has fully embraced the new framework and has already implemented the required data privacy and biosecurity measures to protect our customers and the broader scientific community.
Our Compliance and Attestation
kbDNA has published its attestation of compliance with the new framework, adhering to all six actions outlined for providers and manufacturers. Our commitment includes:
- Publicly stating our adherence to the framework on our website.
- Screening all purchase orders for SOCs utilizing state-of-the-art algorithms and software systems.
- Implementing streamlined customer legitimacy verification processes that validates with efficiency and painlessly for the end-user.
- Retaining detailed records of purchase orders and screening activities for at least three years.
- Ensuring robust cybersecurity measures to protect sensitive data and customer information.
Leading the Industry
Our proactive approach not only ensures compliance but also positions kbDNA as a leader in the industry. We continuously work with regulatory bodies and follow best practices to ensure that our processes remain secure and effective. By choosing kbDNA, you can be confident that you are partnering with a company that prioritizes safety, security, and innovation.
Conclusion
The new Framework for Nucleic Acid Synthesis Screening marks a significant step forward in safeguarding the use of synthetic nucleic acids. At kbDNA, we are committed to upholding these standards and supporting the scientific community in conducting safe and secure research. Together, we can unlock the potential of synthetic biology while mitigating risks and ensuring a safer future.
Contacts & Resources
Relevant Links
2. US Framework for Nucleic Acid Synthesis Screening (“Framework”)
If you have any questions or concerns regarding the Privacy Policy Agreement related to our website, please feel free to contact us at the following email, telephone number or mailing address.
Mailing Address:
KbDNA, INC.
125 Cambridgepark Dr. Suite 301.
Cambridge, MA 02140
Contact Info:
Email: compliance@kbDNA.com
Telephone Number: +1 (781) 206-2235















